Describe What Happens When Ionic and Covalent Molecular Substances Dissolve: A Homework Helper Guide

Have you ever wondered why salt disappears in water, but sugar seems to melt away differently? In this guide, we describe what happens when ionic and covalent molecular substances dissolve. We use simple words to explain these ideas. This helps with homework questions like those on Chegg or Quizlet. We focus on clear answers for school kids and teens. Let’s dive in!
What Is Dissolution?
Dissolution means a substance mixes into a liquid and seems to vanish. Water often acts as the solvent. We call this process the solution formation process. When things dissolve, they spread out evenly. But not all things dissolve the same way. Ionic compounds and covalent compounds act differently. This guide uses a Q&A style to make it feel like homework help. We answer common questions step by step.
Why does this matter? In chemistry class, teachers ask you to explain these differences. It helps you understand aqueous solutions chemistry. We keep things easy, like talking to a friend. No big words unless we explain them.
Question 1: What Are Ionic Compounds?
Ionic compounds form when atoms share electrons. No, wait—they form from ions. Ions are charged particles. Positive ones pair with negative ones. Think of table salt, NaCl. Sodium gives an electron to chlorine. This makes Na+ and Cl-. They stick together in a crystal lattice.
These compounds are hard and brittle. They conduct electricity when melted or dissolved. Why? Because ions move freely then. In solid form, ions stay locked in place.
Examples include:
- Sodium chloride (table salt)
- Potassium nitrate (used in fertilizers)
- Calcium carbonate (in chalk)
Ionic compounds dissolving in water break that lattice. Water pulls the ions apart. This is key to the dissolution of ionic substances.
Question 2: What Are Covalent Molecular Substances?
Covalent compounds share electrons between atoms. They don’t make ions easily. Think of sugar, C12H22O11. Atoms bond tightly without charges.
These are often soft or gases. They don’t conduct electricity well. Why? No free ions.
Examples:
- Water itself (H2O)
- Carbon dioxide (CO2)
- Glucose (sugar)
When covalent molecular substances dissolution happens, molecules stay whole. They mix with water but don’t split. Chegg Homework Help on Dissolution1
Question 3: Why Does Water Dissolve Things?
Water is special. It has the polarity of water. Oxygen pulls electrons more than hydrogen. This makes water molecules like tiny magnets. One end is slightly negative, the other positive.
This polarity helps water grab other particles. For ions, it’s ion-dipole attraction. For molecules, it’s intermolecular forces like hydrogen bonding.
Water acts as a great water as a solvent. It surrounds particles in hydration shells. This keeps them apart and mixed.
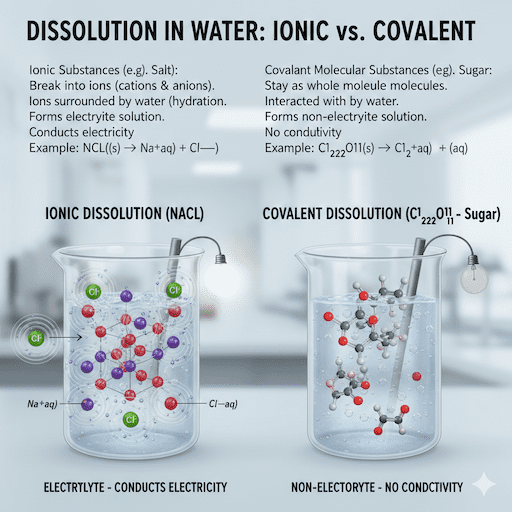
Question 4: Describe What Happens When Ionic and Covalent Molecular Substances Dissolve in Water
Let’s describe what happens when ionic and covalent molecular substances dissolve. For ionic ones, they break into ions. For covalent, they stay as molecules.
Take ionic first. When you add salt to water:
- Water molecules crowd around the crystal.
- Positive ends of water attract negative ions.
- Negative ends attract positive ions.
- This pulls ions away from the lattice.
- Ions get surrounded by water— that’s hydration of ions.
- Now, you have aqueous cations and aqueous anions.
The solution conducts electricity. It’s an electrolytes and nonelectrolytes—wait, electrolyte because of ions.
For covalent, like sugar:
- Water molecules interact with the substance.
- Hydrogen bonding or dipole forces help.
- Molecules separate but don’t ionize.
- They float as whole units in water.
No conductivity here. It’s a non-electrolyte.
This is the big difference between ionic and covalent dissolution.
A Deeper Look at Ionic Dissolution
Let’s break it down more. What happens when ionic compounds dissolve in water?
Imagine NaCl. The crystal lattice breakdown starts. Water’s dipoles attack.
- Step 1: Water touches the surface.
- Step 2: How water separates positive and negative ions—positive Na+ attracts oxygen (negative part of water).
- Step 3: Cl- attracts hydrogen (positive part).
- Step 4: Enough water molecules pull ions free.
- Step 5: Ions move into solution as Na+(aq) and Cl-(aq). That’s aqueous state (aq).
- Step 6: Process repeats until all dissolves.
This is dissociation into ions. Energy from water-ion bonds overcomes lattice energy.
Factors affecting it:
- Temperature: Hotter water dissolves more.
- Stirring: Speeds up mixing.
- Particle size: Smaller pieces dissolve faster.
Examples in life:
- Dissolving salt for cooking.
- Ocean water has dissolved ions.
- Hard water from calcium ions.
Why ionic compounds dissociate in water? Because water’s polarity matches ion charges. Quizlet Explanations on Ionic and Covalent Dissolve2
A Deeper Look at Covalent Dissolution
Now, how covalent molecular substances dissolve?
Sugar is polar, too. It has OH groups for hydrogen bonding with water.
- Step 1: Water surrounds sugar molecules.
- Step 2: Bonds between sugar molecules break.
- Step 3: Water forms new bonds with sugar.
- Step 4: Sugar spreads out as whole molecules.
Not all covalent compounds dissolve. Oil doesn’t—it’s nonpolar. Water can’t grab it.
Do covalent compounds form ions in solution? Usually no. They stay neutral.

Examples:
- Alcohol in water.
- Vinegar (acetic acid, but it ionizes a bit—weak electrolyte).
Why some covalent compounds dissolve in water? If they are polar or can hydrogen bond.
Question 5: What’s the Difference Between Ionic and Covalent Solubility?
Ionic vs covalent solubility boils down to ions vs molecules.
In a table:
| Aspect | Ionic | Covalent |
| What happens | Dissociate into ions | Stay as molecules |
| Conductivity | Yes, the electrical conductivity of solutions | No |
| Examples | Salt, KCl | Sugar, CO2 |
| Forces | Ion-dipole attraction | Intermolecular forces |
| Solution type | Electrolyte | Nonelectrolyte |
This helps in labs. Test the conductivity to tell them apart.
Real-Life Examples and Experiments
Try this at home: Dissolve salt and sugar in water. Use a battery and wires to check conductivity. Salt lights a bulb; sugar doesn’t.
In nature, Rivers dissolve ionic minerals from rocks. That’s why the sea is salty.
In body: Glucose (covalent) dissolves in blood without ionizing.
Step-by-step process of ionic compound dissolving:
- Add solid to water.
- Observe fizzing or heat.
- Stir.
- See a clear solution.
- Test for ions.
For covalent: Similar, but no heat usually.
Common Mistakes in Homework
Students mix up terms. Remember: Ionic = ions, covalent = molecules.
Don’t say covalent dissociates—unless it’s acid.
Use the solvation process for both, but specify the differences.
Advanced But Simple: Energy Involved
Dissolving needs an energy balance. For ionic, lattice energy vs hydration energy.
If hydration > lattice, it dissolves.
For covalent, it’s a weaker force.
But keep it basic for grade school. Quora Discussion on Compounds in Water3 –
Why Learn This?
It explains everyday things. Like, why does detergent clean—mixes oil and water.
In medicine, IV fluids have dissolved ions.
In the environment, Pollution from dissolved chemicals.
FAQs
Explain the dissolution of ionic vs covalent compounds.
Ionic things, like salt, break apart in water. They turn into tiny charged pieces called ions. Covalent things, like sugar, do not break apart. They stay as whole pieces in water. Some covalent things do not mix with water at all.
Describe the hydration of ions in aqueous solution.
When ions are in water, water wraps around them. Water acts like a cozy blanket for each ion. This is called hydration. It helps ions stay apart in the water.
Chemistry explanation for dissolving substances?
Things dissolve if they like the water. Water is polar, like a magnet with two sides. Polar or charged things mix well with water. Non-polar things, like oil, do not mix. Like likes like!
GCSE explanation of ionic and covalent dissolution?
Ionic things dissolve because water pulls the ions out. The ions get wet and move freely in water. Covalent things that are polar, like sugar, mix as whole pieces. Non-polar covalent things do not mix with water.
High school chemistry solution formation explanation?
Water helps make solutions because it is special. It pulls ions apart from ionic things. It mixes with polar pieces from other things. This makes a clear mix called a solution.
Conclusion
To describe what happens when ionic and covalent molecular substances dissolve, remember ionic substances split into charged particles called ions, surrounded by water. Covalent ones mix as whole molecules through bonds with water. This basic idea helps in science class and life.
References
- Chegg Homework Help on Dissolution – Great for fill-in-the-blanks questions. ↩︎
- Quizlet Explanations on Ionic and Covalent Dissolve – Useful for study cards. ↩︎
- Quora Discussion on Compounds in Water – Real people answers. ↩︎





